On behalf of both the Virginia Soybean and Virginia Grain Producers Associations, I invite you to join me for the 2018 Virginia Grain and Soybean Annual Conference on February 20-21, 2018. The conference will span two days and is being held at the Richmond Westin Hotel to provide a convenient, comfortable and inviting environment for attendees and their families. In response to increased interest, this year the conference will have a greatly expanded exhibit hall providing a larger and more prominent space for exhibitors and attendees to network.
Continuing to honor your requests that we not include information that you received at the county and regional meetings, we continue to include exciting, innovative, and largely non-production oriented speakers. Furthermore, following the success of last year’s two-day program, the conference has added even more breakout topics, speakers, and programming to help you run a strong, profitable operation. The program will feature keynote speakers and topics certain to bring value to your operation, including: Smithfield Foods’ Chief Strategy Officer and Chief Commodity Hedging Officer Dhamu Thamodaran; The Port of Virginia’s Mid-Atlantic Area Manager Kara Matzko; and FBI Counterintelligence Training Center Special Agents Mark Betten and Matthew Seckers discussing the topic of intellectual property security and the agriculture industry.
As always, your registration includes all meals including a full dinner that will follow the networking reception Tuesday evening, giving you additional time to network and spend time with colleagues and speakers.
Click Here for Individual Registration
Click Here for Sponsorship Opportunities & Sponsors Registration
What’s on the Agenda?
Tuesday, February 20, 2018
10:30am Registration & Exhibitor Trade Show Opens
11:30am Lunch Buffet Opens
12:00pm Lunch & Commodity Market Speaker
Robert Harper, Grain Division Manager, Virginia Farm Bureau Federation
1:00pm Breakout Sessions – Choose One
Weed & Pest Management in Grain & Soybeans
Charlie Cahoon & Michael Flessner, Virginia Tech
Soil Health Strategies for Increased Yields
Chris Lawrence, NRCS Cropland Agronomist & Dr. Mark Reiter, Eastern Shore AREC
Opportunities & Challenges on the Horizon
Dicamba Update from Monsanto
Rapeseed & Organic Opportunities for a Profitable Rotation, Jeff riddell, Perdue AgriBusiness
2:15pm Break & Visit with Exhibitors
2:30pm Breakout Session Repeated – Choose One
3:45pm Break & Visit with Exhibitors
4:00pm Globalization of Agriculture & Commodities
Dhamu Thamodaran, Chief Strategy Officer and Chief Commodity Hedging Officer of Smithfield Foods
5:00pm Reception & Networking in the Exhibit Hall
6:00pm Awards Dinner
This includes corn and soybean yield contest winner presentations. Although we did not break Keith Brankley’s 2012 Virginia record of 109 bushels per acre, we did induct 3 new members into the 100-bushel club, 3 new members into the 90-bushel club, and 4 new members into the 80-bushel club. Of course David Hula and other corn farmers continue to break yield records in that crop – the number of winners is too large to list in this small space.
Wednesday, February 21, 2018
7:30am Breakfast Buffet Opens
8:00am Annual Meetings, Elections, and Reports
8:30am Updates from Secretary of Agriculture & Forestry Bettina Ring
Break & Visit with Exhibitors
10:00am Managing Security Risks in Agricultural Trade
Special Agent Matthew Seckers, FBI, Richmond Division and Special Agent Mark Betten, Unit Chief for the FBI’s Counterintelligence Training Center, FBI Academy, Quantico, VA
11:00am The Agriculture Industry and the Port of Virginia: Growing New Markets
Kara Matzko, Mid-Atlantic Area Manager, Port of Virginia
12:00pm Dicamba Training & Certification Lunchon
Chelsea Valenti, BASF Crop Protection
Where Can I Stay?
We have reserved a room block at the Richmond Westin at the discounted price of $129 per night. Reservations must be made on or before February 6, 2018 by calling 1-888-627-7786. Reference the “Grain & Soybean Annual Conference” rate. You may also visit:
https://www.starwoodmeeting.com/Book/VGASA2018


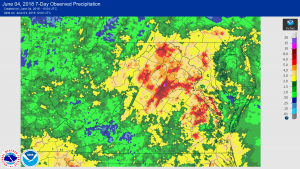





 e to many different factors that range from nutrient deficiencies to cool and wet growing conditions. Take a look at this article to give you a few reasons for this poor looking corn and different things to consider prior to making your sidedress nitrogen applications.
e to many different factors that range from nutrient deficiencies to cool and wet growing conditions. Take a look at this article to give you a few reasons for this poor looking corn and different things to consider prior to making your sidedress nitrogen applications. 