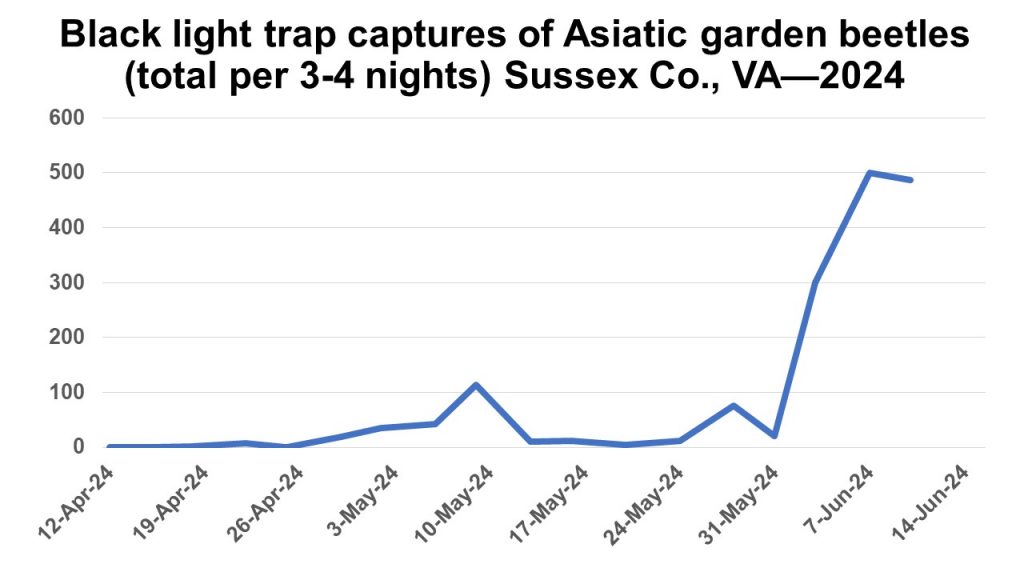
EPA has finally issued a statement on the revocation of tolerances for chlorpyrifos. For this year, 2024, chlorpyrifos can be used on all product labeled crops. In 2025 and beyond, it will only be allowed on 11 specific crops; alfalfa, apple, asparagus, cherry (tart), citrus, cotton, peach, soybean, strawberry, sugar beet, and wheat (spring and winter). However, there will be further state restrictions on those 11 tolerances coming soon (e.g. chlorpyrifos will only be allowed on tart cherries in MI).
———- Forwarded message ———
From: U.S. EPA Office of Chemical Safety and Pollution Prevention oppt.epa@public.govdelivery.com
Date: Fri, Feb 2, 2024 at 1:23 PM
Subject: EPA Update on the Use of the Pesticide Chlorpyrifos on Food
To: dlfrank@vt.edu
EPA Update on the Use of the Pesticide Chlorpyrifos on Food
The U.S. Environmental Protection Agency (EPA) is issuing an update on the use of the pesticide chlorpyrifos on food.
Chlorpyrifos is an organophosphate insecticide used for a large variety of agricultural uses, including soybeans, fruit and nut trees, broccoli, cauliflower, and other row crops, as well as non-food uses. In a final rule issued in August 2021, EPA revoked all tolerances for chlorpyrifos, which establish an amount of a pesticide that is allowed on food. This action effectively stopped the use of the pesticide chlorpyrifos on all food and animal feed. EPA took this action in response to an April 2021 order from the U.S. Court of Appeals for the Ninth Circuit for the Agency to issue—within 60 days—a final rule addressing the use of chlorpyrifos in food or feed crops, without taking public comment or engaging in “further fact-finding.”
That tolerance revocation rule was challenged by a chlorpyrifos registrant and several grower groups in the U.S. Court of Appeals for the Eighth Circuit. On November 2, 2023, the Eighth Circuit issued a ruling vacating EPA’s final rule and sending the issue of chlorpyrifos tolerances back to EPA for further proceedings. The ruling did not include a timeframe or specific instructions for EPA to take a final action on the use of chlorpyrifos in food or feed crops without public comment.
EPA is issuing a technical correction in the Federal Register that changed the Code of Federal Regulations to reflect the Eighth Circuit’s decision. The Eighth Circuit’s mandate issued on December 28, 2023, finalized the court’s judgment and vacated the Agency’s 2021 rule revoking chlorpyrifos tolerances.
Since the tolerances are currently in effect, growers can now use currently registered chlorpyrifos products on all crops with reinstated tolerances, consistent with directions for use on those product labels. However, such uses may be subject to restrictions by individual states.
The Eighth Circuit’s decision stated that EPA should have considered modifying the tolerances in addition to complete revocation and noted that the Agency had “identified 11 specific candidates” of food and feed crop uses whose tolerances could be modified in a Preliminary Interim Decision EPA issued in 2020. Thus, the Agency expects to expeditiously propose a new rule to revoke the tolerances for all but 11 uses with additional restrictions for geographic location and rate of application to address safety of the tolerances, and potential restrictions for farmworker and other vulnerable populations, and vulnerable species and their habitats. Those 11 uses are: alfalfa, apple, asparagus, cherry (tart), citrus, cotton, peach, soybean, strawberry, sugar beet, wheat (spring), and wheat (winter). These 11 uses were identified in the December 2020 Chlorpyrifos Proposed Interim Decision and represented about 55% of the total chlorpyrifos usage (average annual pounds applied) on agricultural commodities between 2014-2018.
EPA is also engaged in discussions with registrants of chlorpyrifos products to further reduce exposures associated with these 11 uses of chlorpyrifos. EPA will also consider the 2020 Proposed Interim Decision and public comments received on that document.
At this time, any existing final cancelation orders, including any terms for sale, distribution, and use of existing stocks of products subject to those cancelation orders and related return programs for chlorpyrifos products, remain in place, unless and until amended by EPA.
EPA will continue to update the public as it evaluates and takes any actions related to chlorpyrifos use.
For more information, view the Federal Register Notice.