Hillary L. Mehl, Extension Plant Pathologist & David Holshouser, Extension Agronomist
The wet weather conditions, along with relatively cool temperatures over the last few weeks have been nearly perfect for disease development in soybean. As always, keep in mind the disease triangle – all 3 conditions must be met before a disease can form.
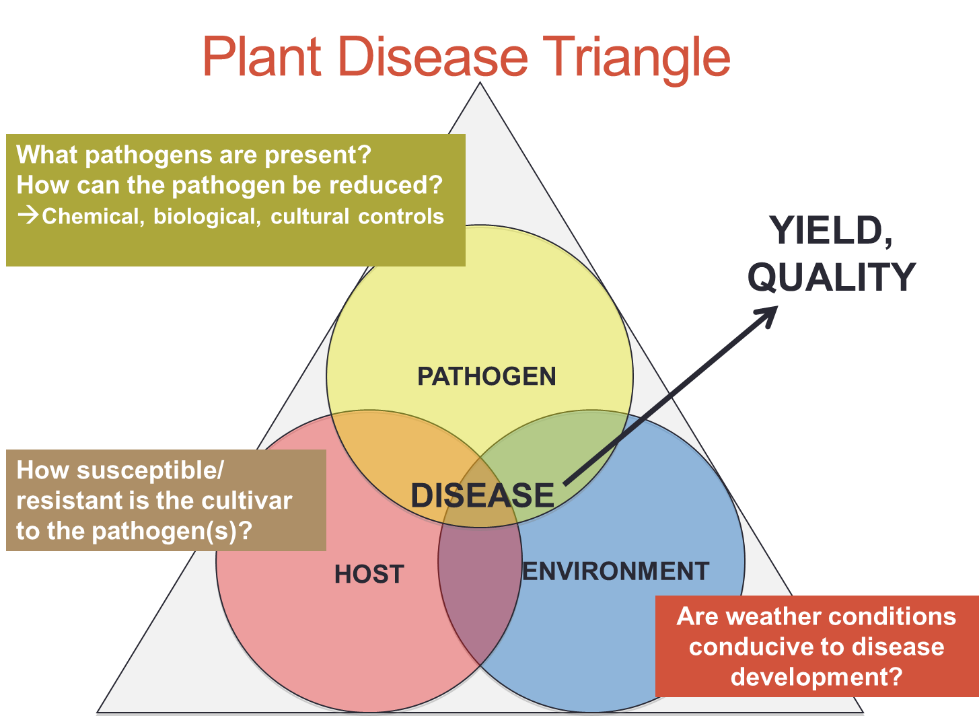
In most cases, one or more soybean pathogens are out there. Refer to previous blogs for more information on common soybean diseases: Now is a Good Time to Evaluate Your Varieties for Foliar Diseases and Foliar Fungicides for Soybeans. You can also access the Mid-Atlantic Soybean Disease Scouting Guide here. For a positive identification of the disease, send leaf samples to our diagnostic lab.

We also need a susceptible soybean variety (host). Some varieties are resistant to specific pathogens; others have some tolerance; many have neither.
Of the 3 conditions, the environment is the most difficult to assess. In general, we need high relative humidity (RH) for an extended period of time, usually over several days, and cool to moderate (e.g. not hot) temperatures.
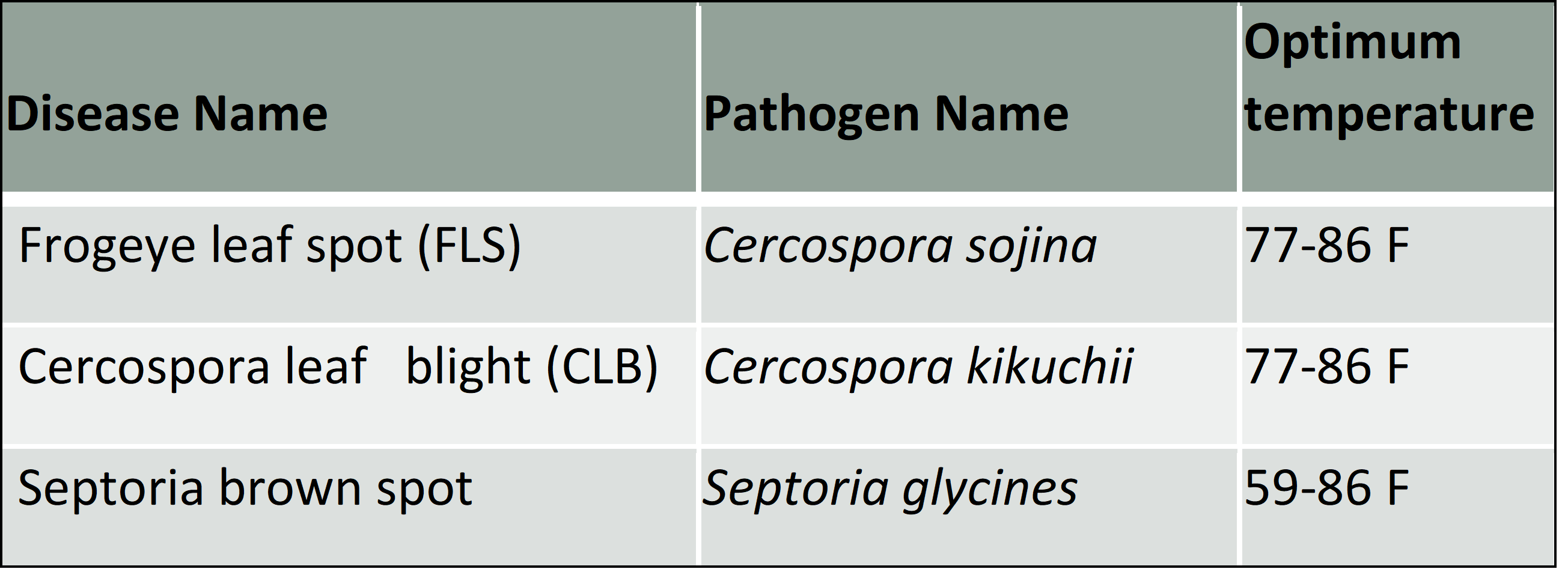
Our research indicates that about 1/3 of the time, a foliar fungicide will result in yield increase. The probability and amount of response will of course depend on the disease. Frogeye leaf spot can be a quite devastating disease in susceptible soybean that have not been rotated. Cercospora leaf blight (sometimes called late blight) is less devastating, but is common in almost all of our soybean when conditions are right.
We have attempted to create a foliar fungicide decision aid that would help decide on whether or not to spray. The decision aid is based on the total number of favorable days for disease development, based on the data shown below.
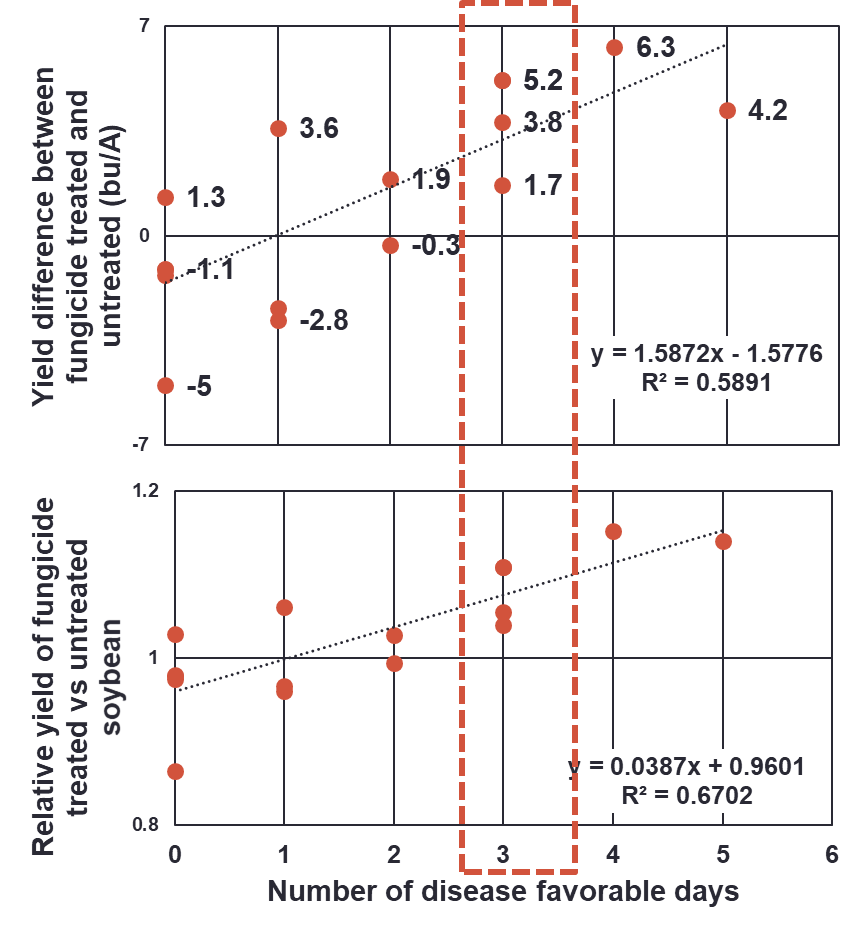
We think that during the period of 1 week before and 1 week after mid-R3, yield-robbing disease levels may develop if we have 3 favorable days with 10 or more hours of relative humidity at or above 95% and temperatures in the optimum range (77 to 86 F). When these conditions are met, we would suggest a fungicide application.
However, after 3 years of on-farm testing we only improved the predictability of a yield increase slightly (approximately 50% versus the 33% we see on average). Why? We have not been able to predict the weather after the fungicide application. If things turn dry, the disease will decline and a yield response is not likely. If conditions remain favorable for disease, then a yield response to a fungicide application is more likely.
So, back to our original question, is it too late to apply a fungicide?
Most of our full-season soybean are past the R3 stage (though many acres were planted in June due to wet weather); so a yield response is not likely if fungicides are applied now. Generally, it takes about 65 to 80 days after planting to reach the R3 stage, depending on planting date and relative maturity.
Double-crop soybean will usually take 40 (MG 4 planted in July) to 60 days (mid- to late-5 planted in June) to reach R3. Since most double-crop soybeans recently entered or will enter the R3 stage, a yield response to a fungicide application is more likely.
What about seed quality? We’ve seen little relationship between an R3 fungicide application and improved seed quality. To insure good seed quality, we would suggest 2 applications (R3 and R5). The R5 application might help with our biggest seed quality issue, Phomopsis seed decay, which tends to develop later in the season. For seed growers, a late application is a good insurance treatment, but keep in mind that if long periods of wet weather delay harvest, seed quality will deteriorate even if a late fungicide was applied. Be aware that most fungicide labels restrict applications once soybean enter the R6 stage. Always follow label instructions.

In summary, there are several things that you need to keep in mind that will affect whether or not you will see a yield response to fungicides:
- Non-rotated soybean will generally have more disease.
- Variety Resistance. Many varieties have very good FLS resistance; some have only moderate resistance – this may work pretty well if soybean are rotated, but don’t depend on it if soybean were grown last year (or many of the previous years). Generally, soybean are not resistant to Cercospora blight, but we have seen differences in varieties. We measure % purple seed stain in our variety tests; however, this is not always a good indicator for resistance to the leaf spot and blight.
- Timing & Soybean Stage. R3 applications are usually best; not always, but most of the time. Still, we occasionally see a response with R5 applications. I (David) have only seen a yield benefit from 2 applications (R3 and R5) once – this was in 80-bushel double-crop soybean in a very wet and cool year.
- Historically, strobilurin fungicides were our most effective on most soybean disease. However, FLS is now largely resistant to that class of fungicides, and control of our other diseases have declined. However, the strobilurins still have utility. We suggest a fungicide containing a strobilurin along with a good triazole. The 2018 soybean fungicide efficacy table can be downloaded here.
- Spray Volume & Droplet Size. Good coverage of fungicide throughout the canopy is necessary. Use at least 15 gallons per acre spray volume and make sure that your nozzles will deliver medium-sized droplets. Small droplets will not penetrate the canopy to the lower leaves, where the fungicide is most needed. Large droplets will not provide uniform coverage. For more detail on this, see Application Equipment for Effective Insect Pests and Foliar Disease Control.
- Seed Quality. Although, we don’t always see a seed quality benefit from an R3 application, two applications should improve seed quality. Furthermore, a late-season (R5) application should help with certain seed diseases such as Phomopsis seed decay. However, keep in mind that if cool, wet conditions delay harvest seed quality will deteriorate even with a late fungicide application. Controlling late season insects such as stink bugs is also critical for preventing fungal infection and maintaining seed quality.